Key Takeaways
A dermal filler consent form template protects your clinic legally and ensures patients understand all risks before treatment
Essential sections include patient medical history, treatment details, risks and complications, contraindications, and aftercare instructions
Consent must be collected before every filler session, not just the first visit, to meet regulatory and insurance requirements
Digital consent forms reduce admin time by up to 40% and eliminate lost paperwork and compliance gaps
Customise your template for specific filler brands (Juvederm, Restylane, Sculptra) to cover product-specific risks
Every aesthetic clinic performing injectable treatments needs a solid dermal filler consent form template. Without one, you expose your practice to legal risk, insurance complications, and patient complaints. However, many clinics still rely on generic consent forms that fail to cover filler-specific risks like vascular occlusion or granuloma formation.
In this article, you will find a comprehensive dermal filler consent form template you can customise for your clinic. Furthermore, we break down each section, explain what regulators expect, and show you how to digitise the entire process.
Download Dermal Filler Consent Form Template
A ready-to-use branded consent form with patient details, medical history, treatment information, risk disclosures, and signature sections.
What Is a Dermal Filler Consent Form?
A dermal filler consent form is a legal document that patients sign before receiving injectable filler treatments. It confirms the patient understands the procedure, its risks, expected outcomes, and alternative options. In addition, it serves as documented proof that the practitioner provided adequate information before treatment.
For example, the Joint Council for Cosmetic Practitioners (JCCP) in the UK requires practitioners to obtain written informed consent for all non-surgical cosmetic procedures. Similarly, the FDA in the United States classifies dermal fillers as medical devices and expects practitioners to disclose all known risks.
Informed consent goes beyond a signature on paper. As a result, your form must demonstrate that the patient had sufficient time to consider the information, ask questions, and make a voluntary decision. Therefore, a well-structured dermal filler consent form template is essential for every clinic offering filler treatments.
Why Your Clinic Needs a Dermal Filler Consent Form Template
Using a standardised dermal filler consent form template brings several benefits to your practice. First, it provides legal protection if a patient later claims they were not informed of potential risks. In contrast, clinics without proper consent documentation face significant liability exposure.
Legal Protection
Malpractice claims related to cosmetic injectables have increased by over 30% in the past five years. A properly completed consent form is your primary defence in any dispute. For instance, if a patient develops an adverse reaction, your documented consent proves they were warned about that specific risk.
Patient Safety
Consent forms serve as a clinical safety net. They prompt practitioners to review medical history, check for contraindications, and discuss potential complications. Consequently, this structured approach reduces the risk of treating patients who may be unsuitable candidates for dermal fillers.
Insurance and Compliance
Most professional indemnity insurers require documented informed consent for every injectable procedure. Furthermore, compliance management frameworks like CQC in the UK explicitly assess consent processes during inspections. Similarly, HIPAA-compliant practices in the US must securely store all consent records.
Building Trust
Patients who feel informed and respected are more likely to return for future treatments. In addition, a professional consent process demonstrates your clinic takes patient welfare seriously. As a result, your reputation and rebooking rates both improve.
Key Sections of a Dermal Filler Consent Form
A thorough dermal filler consent form template should include the following sections. Each one plays a specific role in protecting both the patient and the practitioner.
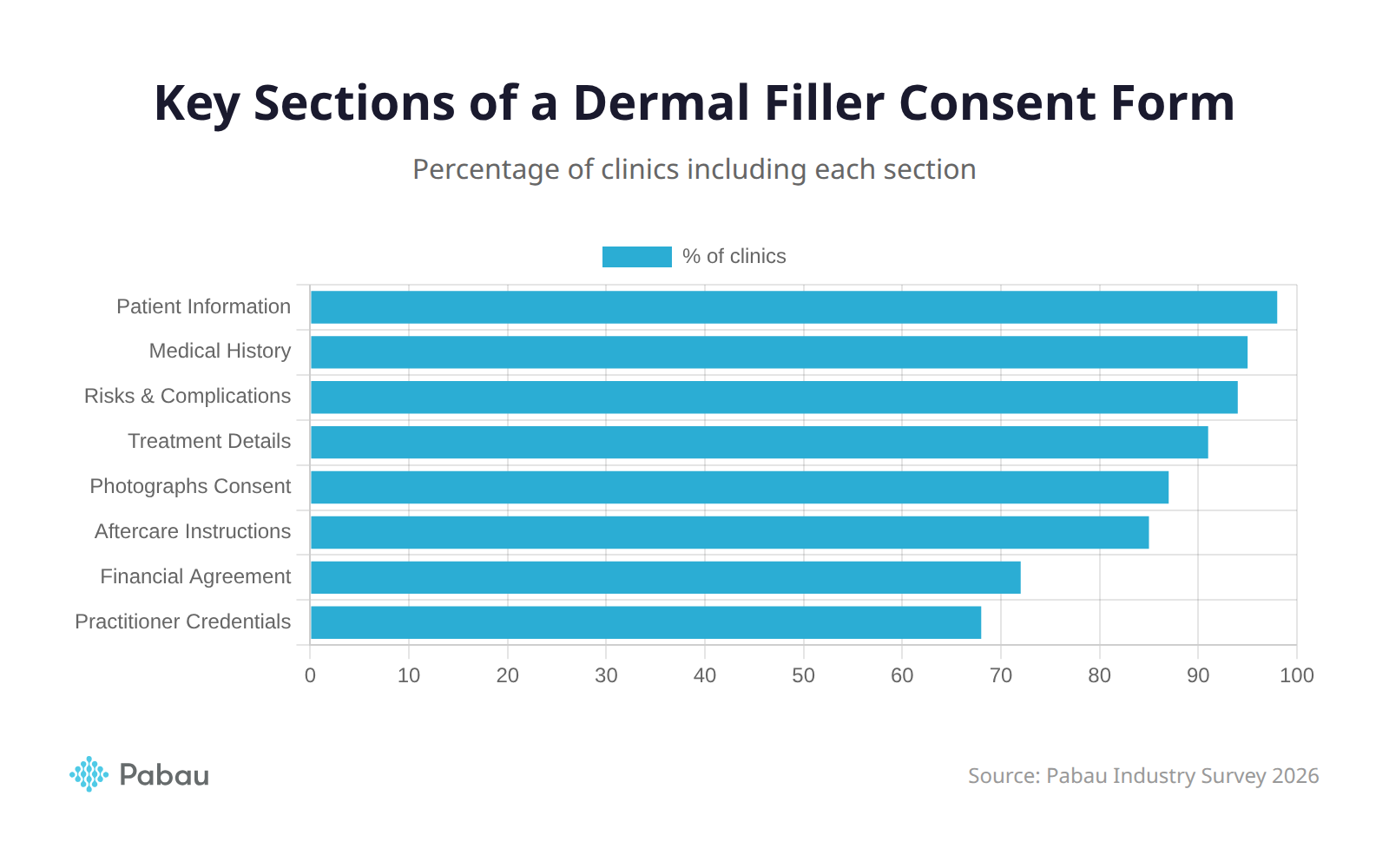
Patient Information and Medical History
This section captures the patient’s full name, date of birth, contact details, and comprehensive medical history. For example, you should ask about autoimmune disorders, bleeding conditions, pregnancy or breastfeeding status, and any history of cold sores (herpes simplex). In addition, document current medications including blood thinners, NSAIDs, and supplements that may increase bruising risk.
Treatment Details and Expected Outcomes
Clearly outline the specific filler product being used (e.g. Juvederm Voluma, Restylane Lyft), the injection sites, the volume to be administered, and realistic expected outcomes. However, always emphasise that results vary between individuals. Furthermore, include information about the expected longevity of results and when the patient may need a follow-up treatment.
Risks, Side Effects, and Complications
This is the most critical section of your dermal filler consent form template. List both common and rare complications, including:
- Bruising, swelling, and redness at injection sites
- Asymmetry or overcorrection
- Infection at the injection site
- Allergic reactions to filler components
- Nodule or granuloma formation
- Vascular occlusion (potentially leading to tissue necrosis or blindness)
- Migration of filler material
Pro Tip
List vascular occlusion and blindness risk explicitly on every dermal filler consent form. These rare but serious complications are the most common basis for malpractice claims related to fillers.
Contraindications and Allergies
Document known contraindications including active skin infections, autoimmune conditions, pregnancy, breastfeeding, and known allergies to filler ingredients (e.g. lidocaine, hyaluronic acid). As a result, this section acts as a clinical screening tool alongside the consultation form.
Photographs and Record Consent
Many clinics take before and after photos for clinical records and marketing. Consequently, your consent form should include a separate section requesting permission to photograph the patient. In addition, specify whether photos may be used for marketing or educational purposes.
Aftercare Instructions
Include a summary of post-treatment care instructions within the consent form. For example, advise patients to avoid strenuous exercise for 24 hours, not to touch the treated area, and to report any unusual symptoms immediately. Therefore, patients cannot claim they were not informed about aftercare.
Practitioner Information and Credentials
List the treating practitioner’s full name, qualifications, registration number, and insurance details. This transparency builds patient confidence. Similarly, it demonstrates medspa compliance with regulatory requirements.
Patient Signature and Date
The final section confirms the patient has read and understood all information, has had the opportunity to ask questions, and voluntarily consents to treatment. In addition, include a separate signature line confirming the cooling-off period was offered.
Dermal Filler Consent Form Template
Below is a customisable dermal filler consent form template. Adapt each section for your clinic’s specific needs and filler brands.
Section 1: Patient Details
- Full name, date of birth, address, contact number, email
- Emergency contact details
- GP or primary care provider name and address
Section 2: Medical History Questionnaire
- Current medications (including supplements)
- Known allergies (especially to lidocaine or hyaluronic acid)
- Previous cosmetic treatments and any adverse reactions
- Autoimmune conditions, bleeding disorders, pregnancy/breastfeeding status
- History of cold sores or facial herpes simplex
Section 3: Treatment Information
- Filler product name, brand, and batch number
- Injection sites and volumes
- Expected results and duration
- Alternative treatment options discussed
Section 4: Risks and Complications Disclosure
- Full list of potential side effects (common and rare)
- Specific mention of vascular occlusion risk
- Statement that no guarantee of results is made
Section 5: Consent Declarations
- “I confirm I have read and understood the above information”
- “I have had the opportunity to ask questions”
- “I understand the risks and possible complications”
- “I consent to before and after photographs for clinical records”
- “I consent to / decline the use of photographs for marketing”
Section 6: Cooling-Off Period
- Statement confirming the patient was offered a cooling-off period
- Date consent was first provided vs date of treatment
Section 7: Signatures
- Patient signature and date
- Practitioner signature, name, qualifications, and date
Pro Tip
Always record the filler batch number on the consent form. If a product recall occurs, you can quickly identify affected patients and contact them directly.
Customising for Specific Filler Brands
Different filler brands carry different risk profiles. For instance, Sculptra (poly-L-lactic acid) has specific risks around nodule formation that differ from hyaluronic acid fillers. Similarly, calcium hydroxylapatite fillers (Radiesse) cannot be dissolved with hyaluronidase. Therefore, add brand-specific risk sections to your template when using these products.
How to Use a Dermal Filler Consent Form in Your Practice
Having a template is only the first step. How you implement it determines whether it truly protects your clinic.
When to Collect Consent
Collect fresh consent before every treatment session, not just the first visit. For example, a patient returning for lip filler top-ups six months later should complete a new form. Their medical history, medications, or health status may have changed. In addition, regulatory guidance recommends re-consenting when changing filler products or treatment areas.
Verbal Explanation and Cooling-Off Period
The consent form supplements a face-to-face discussion, not replaces it. Walk through each section verbally during the client consultation. Furthermore, offer a cooling-off period (minimum 24 hours for first-time patients) between the consultation and the treatment. This is a JCCP recommendation and a requirement under some UK insurance policies.
Storing and Managing Consent Records
Paper consent forms get lost, damaged, or misfiled. However, digital forms solve this problem entirely. Store all consent records securely with the patient’s clinical file. In addition, ensure records are accessible for at least 10 years (the typical limitation period for personal injury claims in the UK).
“Since switching to digital consent forms with Pabau, our clinic runs so much more smoothly. Patients complete everything before they arrive, and we never worry about missing paperwork or compliance gaps. It has genuinely transformed how we manage our injectable treatments.”
Common Mistakes to Avoid with Filler Consent Forms
Even experienced clinics make errors with their consent documentation. Avoid these common pitfalls to strengthen your legal position.
Using generic consent forms. A general medical consent form does not cover filler-specific risks like vascular occlusion or product migration. Always use a dedicated dermal filler consent form template.
Missing specific risk disclosures. Failing to mention rare but serious complications (blindness, tissue necrosis) leaves your clinic vulnerable. Courts have ruled against practitioners who omitted low-probability but high-severity risks.
Not updating for new products. When you introduce a new filler brand, update your consent form to reflect its specific composition and risk profile. For instance, switching from hyaluronic acid to calcium hydroxylapatite fillers requires different risk disclosures.
Relying on paper-only processes. Paper forms create compliance gaps. They can be lost, illegible, or incomplete. In contrast, digital systems flag missing fields and prevent submission until all sections are complete.
Skipping re-consent for returning patients. A consent form from six months ago does not cover today’s treatment. Medical history changes, new medications appear, and regulations evolve. Therefore, always collect fresh consent.
Going Digital with Your Dermal Filler Consent Forms
Paper consent forms are increasingly outdated. Digital solutions offer significant advantages for aesthetic clinics managing high volumes of injectable treatments.
Benefits of Digital Consent Forms
Digital dermal filler consent form templates reduce admin time, eliminate lost paperwork, and ensure every field is completed. For example, mandatory fields prevent patients from skipping the medical history section. In addition, digital forms integrate directly with patient records for seamless documentation.
How Pabau Automates Consent Collection
Pabau’s capture forms allow you to build custom dermal filler consent form templates that match your exact requirements. Patients receive their forms via the client portal before their appointment. As a result, they arrive having already reviewed risks, completed their medical history, and signed digitally.
Furthermore, Pabau stores every signed consent form securely within the patient’s record. You can retrieve any form instantly during an audit or inspection. Similarly, injection plotting tools let you map exactly where fillers were placed, creating a complete treatment record alongside the consent.
Ensuring Compliance at Scale
For multi-location clinics, digital consent ensures consistency across every site. However, managing medical forms manually becomes impossible at scale. Automated reminders ensure patients complete forms before arrival, and version control guarantees everyone uses the latest template. Consequently, your GDPR compliance and data security improve significantly.
Expert Picks
Building a Botox treatment form too? See our Botox treatment form template guide for a ready-to-use template with injection mapping.
Need a broader consultation workflow? Our med spa consultation form template covers the full intake process beyond consent.
Want to map injection sites digitally? Learn how injection plotting creates visual treatment records alongside your consent documentation.
Ready to Digitise Your Dermal Filler Consent Forms?
Join thousands of aesthetic clinics using Pabau to automate consent collection, store signed forms securely, and stay compliant without the paperwork. Custom templates, digital signatures, and seamless integration with your patient records.
Frequently Asked Questions
A dermal filler consent form template should include patient details, medical history, treatment information (filler brand, volume, injection sites), a full list of risks and complications, contraindications, photograph consent, aftercare instructions, and patient and practitioner signatures.
Yes. Best practice requires fresh consent before every dermal filler treatment session. The patient’s medical history, medications, or health status may have changed since their last visit. Regulatory bodies like the JCCP recommend re-consenting for each procedure.
In the UK, the JCCP recommends a minimum 24-hour cooling-off period for first-time cosmetic patients. While not always legally mandatory, offering a cooling-off period strengthens your consent process and demonstrates good clinical practice. Many insurers also require it.
Absolutely. Digital consent forms are legally valid and offer significant advantages over paper. They ensure all fields are completed, integrate with patient records, and provide secure storage. Platforms like Pabau’s digital forms allow patients to complete and sign consent before their appointment.
In the UK, retain consent forms for at least 10 years, which aligns with the limitation period for personal injury claims. In the US, retention requirements vary by state but typically range from 7 to 10 years. Digital storage makes long-term record keeping straightforward and compliant.
If a patient declines to sign a dermal filler consent form, you should not proceed with treatment. Document the refusal in their clinical notes, explain why consent is necessary, and offer them time to reconsider. Treating without documented consent exposes your clinic to serious legal liability.