Key Takeaways
A hydrafacial consent form documents patient understanding of the procedure, risks, contraindications, and alternatives before treatment begins.
Essential sections include patient details, treatment description, risks and side effects, contraindications, alternatives, and dual signatures.
Consent must be collected before every hydrafacial session to satisfy regulatory requirements and protect your clinic legally.
Digital consent forms reduce admin time, eliminate lost paperwork, and keep your clinic compliant with GDPR and data protection standards.
Every aesthetic clinic offering hydrafacial treatments needs a properly structured hydrafacial consent form. Without one, you risk legal exposure, insurance complications, and regulatory non-compliance. Yet many clinics still use generic facial consent forms that fail to address hydrafacial-specific risks like serum allergies or post-extraction purging.
In this article, you will find a free, downloadable hydrafacial consent form template designed for UK and international clinics. We also break down each section, explain what to include, and show you how to streamline the process with digital consent forms.

Download Hydrafacial Consent Form Template
A ready-to-use branded consent form with patient details, treatment description, risks, contraindications, alternatives, and signature sections.
What Is a Hydrafacial Consent Form?
A hydrafacial consent form is a legal document that patients sign before undergoing a hydrafacial treatment. It confirms that the patient understands the procedure, its risks, expected outcomes, and available alternatives. In addition, it provides documented proof that the practitioner delivered adequate information and obtained voluntary agreement.
The Joint Council for Cosmetic Practitioners (JCCP) requires written informed consent for all non-surgical cosmetic procedures performed in the UK. Likewise, the American Med Spa Association (AmSpa) recommends treatment-specific consent documentation for every aesthetic service.
In the US, the HydraFacial delivery system is classified by the FDA as a Class I exempt medical device, meaning it is subject to general controls but does not require 510(k) premarket clearance. This classification reflects the device’s low-risk profile, though practitioners remain responsible for ensuring patients understand the treatment and provide informed consent.
Informed consent is more than a signature. Your hydrafacial consent form must demonstrate that the patient had enough time to consider the information, ask questions, and reach a voluntary decision. Therefore, a well-structured form is essential for every clinic offering this treatment.
Why Your Clinic Needs a Hydrafacial Consent Form
Using a dedicated hydrafacial consent form brings measurable benefits to your practice.
Legal Protection
A completed consent form is your primary defence in any dispute. If a patient experiences an adverse reaction, documented consent proves they were warned about that specific risk. Generic facial consent forms often lack hydrafacial-specific language, which weakens your legal position.
Patient Safety
The form prompts practitioners to review medical history, check contraindications, and discuss complications before treatment. This structured approach reduces the likelihood of treating unsuitable candidates.
Regulatory Compliance
Professional indemnity insurers require documented informed consent for aesthetic procedures. GDPR-compliant clinics must also demonstrate lawful processing of the health data collected through consent forms. In the US, HIPAA compliance applies to any practice handling protected health information.
Building Patient Trust
Patients who feel informed are more likely to return for future sessions. A professional consent process signals that your clinic prioritises safety and transparency.
Key Elements of a Hydrafacial Consent Form
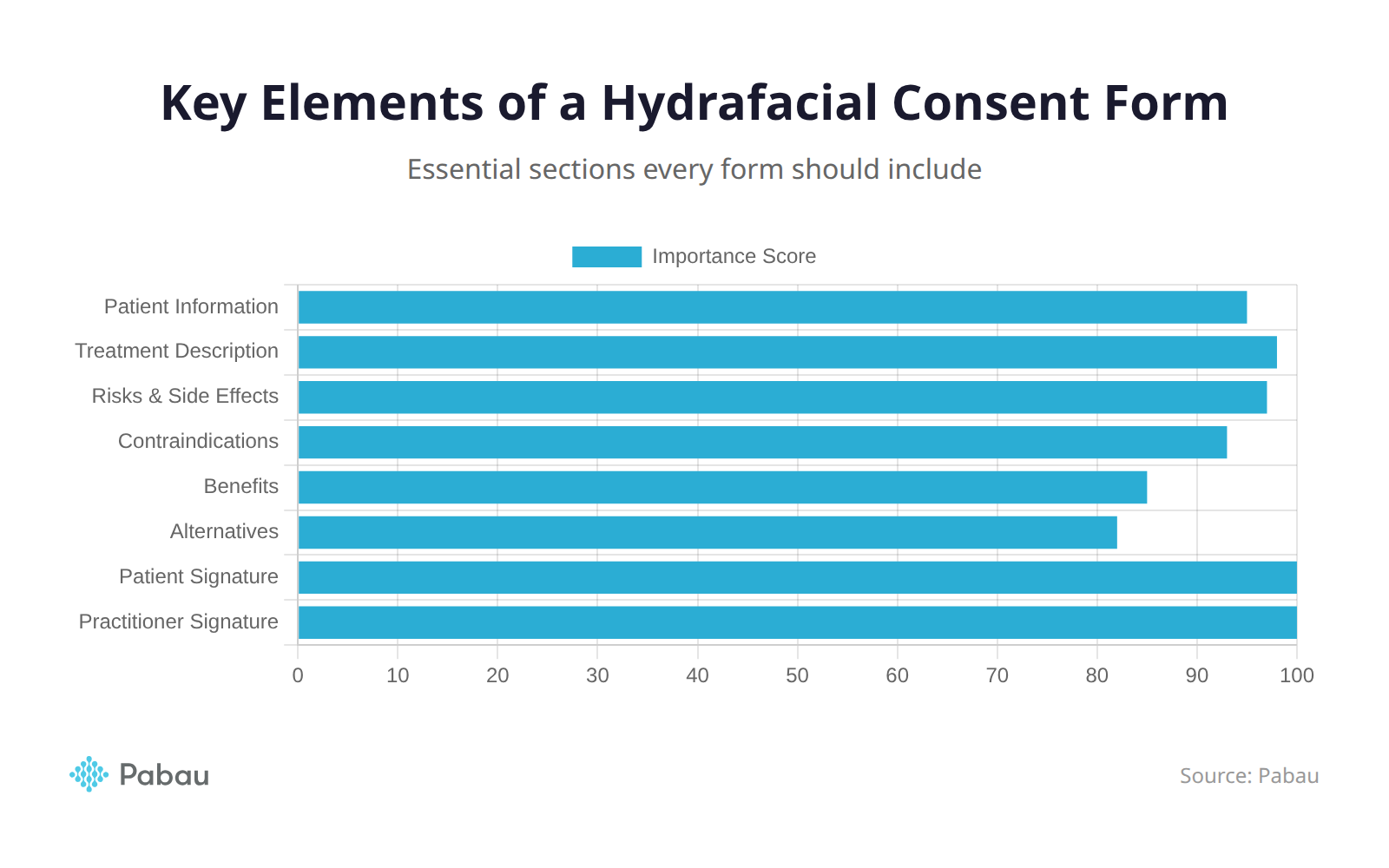
Every hydrafacial consent form should include the following sections.
Patient Information
Collect the patient’s full name, date of birth, contact details, known allergies, current medications, and relevant medical history. This information allows the practitioner to assess suitability and identify potential contraindications. Recording the patient’s Fitzpatrick skin type (I to VI) is also recommended, as higher skin types carry an increased risk of post-inflammatory hyperpigmentation following chemical exfoliation or extraction.
Treatment Description
Explain what a hydrafacial involves. The form should describe the multi-step process: cleansing and exfoliation, gentle acid peel, vortex extraction of impurities, and infusion of antioxidants, peptides, and hyaluronic acid. Patients must understand exactly what will happen during their session.
Risks and Side Effects
List all potential risks clearly. Common side effects include temporary redness or flushing, mild skin sensitivity, tightness, and temporary breakouts (particularly after extractions). Less common risks include allergic reactions to serums, bruising, swelling, or prolonged irritation.
Pro Tip
List each risk as a separate bullet point rather than burying them in a paragraph. Patients are more likely to read and understand a bulleted list, and it strengthens your legal position if a dispute arises.
Contraindications
Your hydrafacial consent form must clearly state conditions that make treatment unsuitable. These include active skin infections (herpes simplex, impetigo), rosacea flare-ups, recent chemical peels or laser treatments within two weeks, current or recent isotretinoin (Accutane) use (manufacturer and practitioner guidance varies, but a minimum of three to six months post-cessation is widely recommended), sunburn, open wounds in the treatment area, and pregnancy.
For patients with a history of skin sensitivities or allergies, a patch test with the intended serums should be performed at least 24 hours before the first treatment. Include a checkbox or field on the consent form to record whether a patch test was offered and its outcome.
Benefits
Outline expected benefits: improved skin texture and tone, reduced fine lines, minimised pores, increased hydration, reduced hyperpigmentation, and enhanced radiance. Include a statement that results vary between individuals.
Alternatives
Mention alternative treatments that may achieve similar results. These include manual facials, chemical peels, microdermabrasion, dermaplaning, and LED light therapy. This section satisfies the informed consent requirement to present all reasonable options.
Signatures
The form must include spaces for the patient’s signature, printed name, and date. Equally important is the practitioner’s declaration confirming they explained the treatment, risks, and alternatives. The practitioner must also sign and date the form.
How to Use the Hydrafacial Consent Form
The person obtaining consent should be the practitioner who will perform the treatment, or someone equally qualified to perform it and answer clinical questions. In the UK, this aligns with JCCP and GMC guidance that the consent-taker must be capable of explaining the procedure, its risks, and alternatives from clinical experience.
Follow these steps to integrate the hydrafacial consent form into your clinic workflow.
Step 1: Send the form before the appointment. Use your clinic management software to send the consent form digitally via email or patient portal at least 24 to 48 hours before the session. The JCCP recommends a cooling-off period between information delivery and consent for cosmetic procedures, giving patients adequate time to read, consider, and ask questions before attending.
Step 2: Review responses on arrival. When the patient arrives, review their completed form. Check medical history, allergies, and medications for any contraindications.
Step 3: Discuss the treatment in person. Walk through the procedure, risks, and alternatives verbally. Assess whether the patient has the capacity to consent and that their motivations and expectations are realistic. The JCCP and Mental Health Foundation joint guidance recommends that practitioners consider the patient’s psychological readiness for cosmetic procedures. Ask if the patient has any questions and document that this conversation took place.
Step 4: Obtain the signature. The patient signs to confirm understanding and consent. The practitioner countersigns with their own declaration.
Step 5: Store securely. File the signed consent form in the patient’s clinical record. Digital forms stored within practice management software like Pabau ensure compliance with data protection regulations.
Hydrafacial Consent Form: Risks and Contraindications in Detail
Understanding risks in depth helps practitioners explain them confidently to patients. Here is a detailed breakdown.
Common Side Effects
Temporary redness typically subsides within 30 minutes to two hours. Mild sensitivity and tightness are normal responses to exfoliation and extraction. Post-treatment breakouts can occur as the skin purges impurities drawn to the surface during extraction.
Uncommon Risks
Allergic reactions to the serums or booster solutions used during treatment are rare but possible. Patients with known sensitivities should request a patch test beforehand. Bruising from suction is uncommon but may occur in patients with fragile skin or those taking blood-thinning medications.
Contraindication Details
Active herpes simplex can be triggered by facial treatments, potentially causing a cold sore outbreak. Patients on or recently off isotretinoin have thinned, sensitised skin that is more susceptible to damage from exfoliation and suction. The required waiting period after cessation varies by practitioner and device manufacturer guidance, typically between three and six months for non-ablative treatments. Recent chemical peels or laser resurfacing leave the skin barrier compromised, increasing the risk of irritation and adverse reactions.
Legal and Compliance Considerations
UK Regulations
The JCCP and Health Education England guidelines require documented informed consent for non-surgical cosmetic procedures. For clinics registered with the Care Quality Commission (CQC), consent processes are assessed during inspections, and failure to demonstrate proper documentation can result in enforcement action. Most non-surgical aesthetic clinics are not currently required to register with CQC, though the UK government’s forthcoming licensing scheme for non-surgical cosmetic procedures is expected to change this.
For a complete overview of UK regulatory requirements, see our aesthetic clinic compliance checklist.
Data Protection
Consent forms contain sensitive health data. Under GDPR, you must have a lawful basis for processing this data, store it securely, and provide patients with access upon request. Digital consent forms within a compliant system like Pabau simplify this process. Similarly, US-based clinics must meet HIPAA requirements for storing patient health information.
Record Retention
In the UK, the NHS Records Management Code of Practice recommends retaining clinical records for a minimum of eight years after the last interaction (or until the patient turns 25, whichever is longer, for minors). In the US, retention requirements vary by state, typically ranging from seven to ten years after the last patient contact. Check your professional indemnity or malpractice insurance policy, as some insurers require longer retention periods. Digital storage eliminates the risk of lost or damaged paper forms.
Digitising Your Hydrafacial Consent Form
Paper consent forms create administrative burden and compliance risks. Digital forms offer significant advantages.
Patients complete forms on their own device before arriving at your clinic. This reduces wait times and ensures nothing is missed. Completed forms sync directly to the patient’s record, eliminating manual filing. Automated reminders prompt patients who have not yet completed their forms.
With Pabau, you can build custom digital intake and consent forms that match your clinic’s branding. Forms are stored securely, linked to patient records, and accessible from any device.

Expert Picks
Need a consent form for fillers? Dermal filler consent form template with product-specific risk disclosures.
Offering microneedling too? Microneedling consent form template covering needle depth and contraindications.
Running a medical spa? Key requirements for a successful medical spa including compliance essentials.
Choosing the Right Consent Form Format
When selecting a format for your hydrafacial consent form, consider both practical and compliance factors. Paper forms are familiar but create storage challenges, risk damage or loss, and slow down the check-in process. PDF templates work well as a starting point but still require printing, scanning, and manual filing.
Digital consent forms offer the strongest combination of efficiency and compliance. They auto-populate patient details from existing records, enforce mandatory fields so nothing gets skipped, timestamp every signature for audit purposes, and sync completed forms directly to the patient’s clinical file. For clinics performing multiple treatments per day, digital forms save significant administrative time while maintaining a complete, searchable audit trail.
Digitise Your Clinical Forms
See how clinics use Pabau to create, customise, and automate clinical forms and templates.
Frequently Asked Questions
In the UK, the JCCP requires written informed consent for all non-surgical cosmetic procedures. In the US, state regulations and professional guidelines vary, but documented consent is considered standard of care and is typically required by malpractice insurers.
Yes. Best practice is to obtain fresh consent before each treatment session. Medical history, medications, and skin conditions can change between appointments, and updated consent ensures accurate risk assessment every time.
Key contraindications include active skin infections (herpes simplex, impetigo), rosacea flare-ups, recent chemical peels or laser treatments, current or recent isotretinoin use, sunburn, open wounds, and pregnancy. Your form should list each one clearly.
A generic facial consent form is not recommended. Hydrafacials involve specific technology (vortex suction, acid peels, serum infusion) with unique risks that a general form does not cover. Treatment-specific consent strengthens your legal protection and improves patient understanding.
In the UK, the NHS Records Management Code of Practice recommends a minimum of eight years after the last interaction. For minors, keep records until the patient turns 25. In the US, state requirements vary from seven to ten years. Check your professional indemnity or malpractice insurance policy for specific retention requirements, as some insurers require longer periods.